My research interests fall under the categories:
Biomineralization in general; formation/secretion principles of carbonate biological hard tissues
Biomineralized tissue adaptation to environments, changes of habitats and climates
Biodiversity and biomineralization principles
Microstructure, texture, material properties characterization of modern and fossil biological hard tissues
Biomineralized tissue repair by the organisms living in diverse habitats
Influence of polymers (biopolymers, bacterial EPS, hydrogels) on crystallite/mineral organization and polymer-mineral composite formation
Transfer of knowledge between biological and biomimetic material fabrication principles
Research is carried out employing the following analytical techniques: biochemical preparation for cell biological studies, electron microscopy, diffraction methods (EBSD, XRD), AFM, confocal laser light microscopy, micro- and nanoindentation.
Current research projects led by Erika Griesshaber are outlined below.
Biomineralization strategies of single- and multicelled organisms
Biomineralization: biologically-mediated material formation, is the controlled formation of mineralized tissue resulting from biological activity. Biomineralizing organisms produce a wide range of molecules that interact and create hierarchically organized organic–inorganic composite materials. When producing these two major components, biopolymers and biominerals, biomineralizing organisms exert strong control over most biomineral characteristics such as composition, biocrystal morphology/orientation, material properties by varying physicochemical secretion conditions, such as osmotic pressure, temperature, pH, biofluid chemistry. The resulting bio-composite product is a low-cost material with exceptional mechanical, optical and magnetic properties. Evolution throughout the geological record created vast biodiversity and a wide range of biomineralized materials. Biological material formation is intrinsically connected to the living organism; the central game players are living cells. Even though our environment is populated by a large variety of animal/plant taxa, organisms exhibit three major basic motives for material generation: (i.) interaction and transport between extracellular and intracellular environments, (ii.) synthesis of components within cells, (iii.) deposition of components within and outside cells. Research of the last decades has shown that various biomineralization strategies were and are active that lead to the different forms of biomineralizing systems: (i) intracellular, within vesicles (coccolithophores), (ii) extracellular (bivalves, gastropods, brachiopods, corals), (iii) within syncytia (echinoderms), (iv) secreted by cell extensions, pseudopodia (foraminifera).
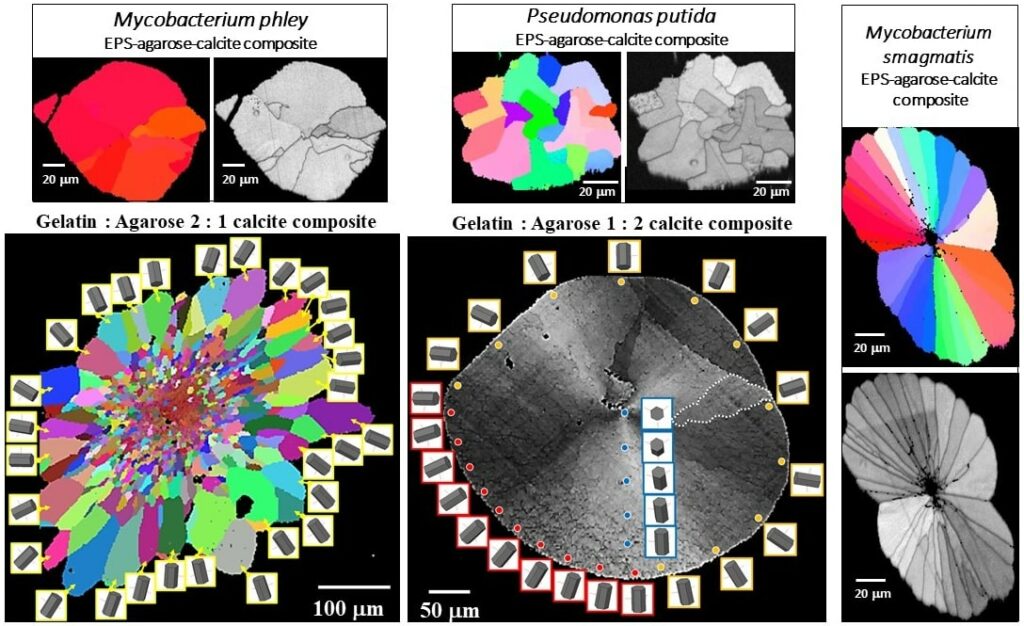
Effect of biopolymers in biomaterial formation and organization
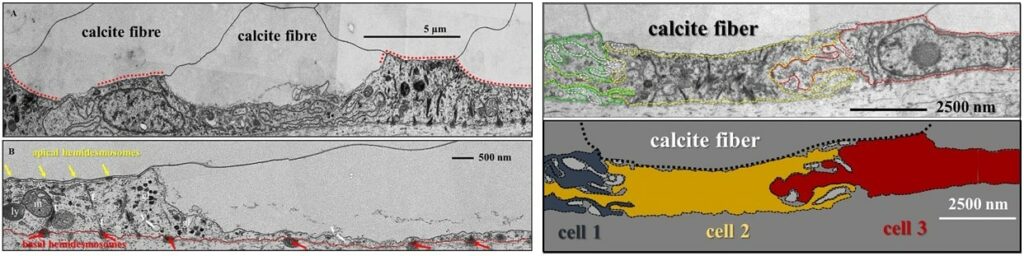
Mineralized structures generated under biological control are hierarchical composites with a large variety of structural design concepts. They consist of two distinct types of materials: (1) a compliant biopolymer matrix that is reinforced by (2) stiff and hard components, such as Ca-carbonate or Ca-phosphate minerals. In gastropod, bivalve and brachiopod shells the extracellular matrix is formed of two major components: a structural and a functional component. Matrix membranes are arranged in a characteristic grid or honey-comb structure, subdivide space and influence the shape and size of the biocrystals that comprise the biological hard tissue. Scaffold (matrix) membranes are porous and enable the transfer of crystallographic orientation information from one compartment into the other. This facilitates the formation of mesoscale entities consisting of a few co-oriented mineral units, as, for example, is the case in both shell layers of the bivalve Mytilus edulis Griesshaber et al. 2013 or in the fibrous shell layer of the brachiopod Megerlia truncata Schmahl et al. 2008.
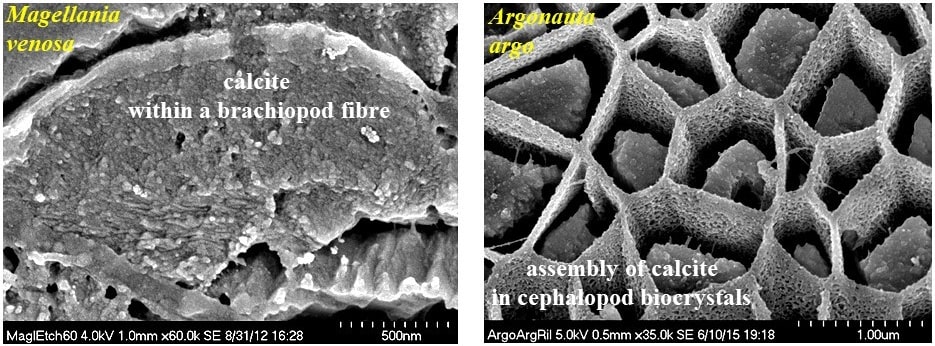
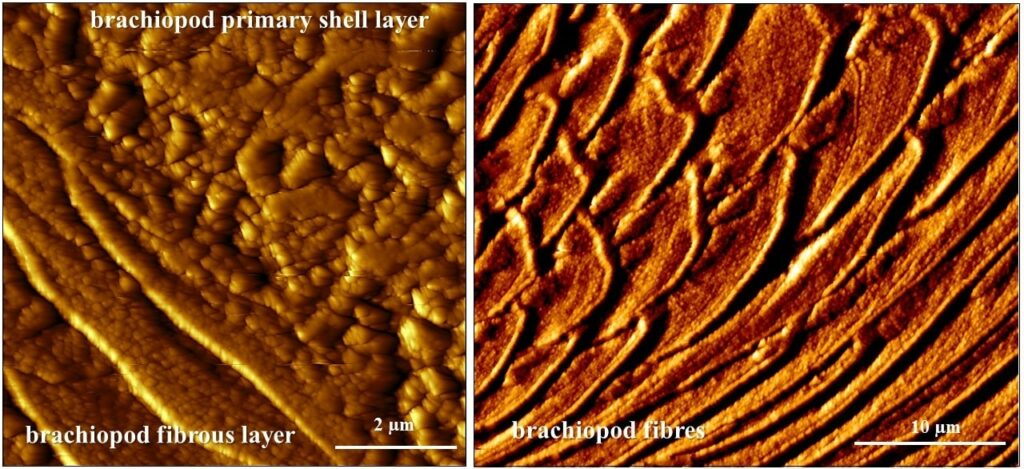
Functionality of carbonate biological hard tissues
Organization of nanosized entities across many length scales poses a major challenge in the development and production of man-made materials with advanced functions. In contrast, in biologically formed hard tissues, this design feature and its formation principle is intrinsic. It began already with the emergence of first skeletal hard parts in late Precambrian and was diversified since then by evolutionary adaptation. For living organisms biological activity is geared to optimize material characteristics for many purposes such as adaptation, functionality, predation avoidance, survival in habitats. A vast diversity of biocrystal organizational patterns exist for biological hard tissue, these range from almost unaligned to highly co-aligned crystal assemblies. Hence, with the evolution of biological materials, both a high order as well as a high disorder in mineral organisation may be advantageous for the organism. In contrast to man-made materials all biologically fabricated materials/tissues have a hierarchical architecture. Deciphering design principles of biomineralized composites is a prerequisite for manufacturing biomimetic analogues of novel materials with targeted and application-specific properties.
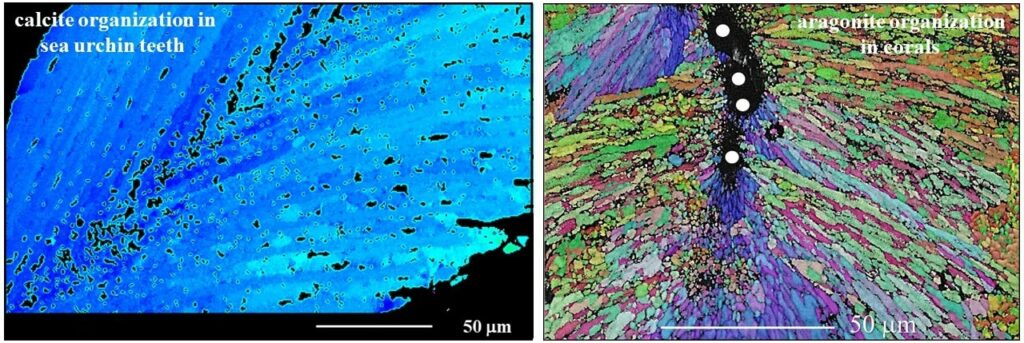
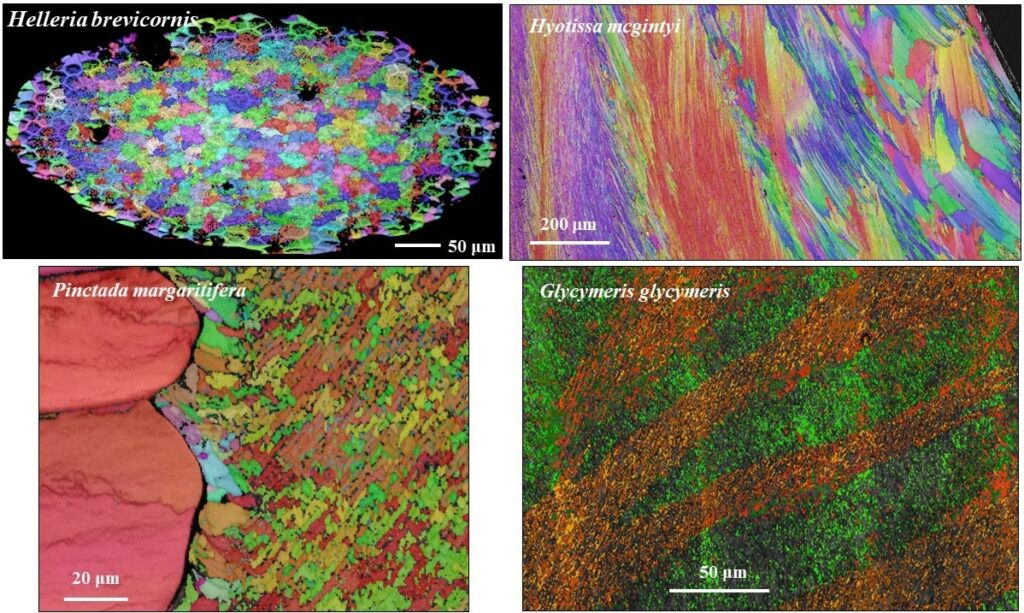
Microstructure, texture and material function relationships
Biological structural materials can be regarded as being a coupled function of structural arrangement on different hierarchical levels connected to individual constituent chemistries and material characteristics. This enables the generation of composite materials with specific and outstanding material properties. The staggered arrangement of biocrystals in biopolymer matrices, the nanocomposite nature of the mineral phase, the geometry, the aspect ratio of the biocrystals (e.g. fibers, platelets, prisms), the ability of the polymer phase to dissipate deformation, the presence of extensive interfaces between biopolymer and mineral components and the hierarchical architecture of the composite structural material enable the formation of biological structural materials with exquisite optical and material properties. Organization of nanosized entities across many length scales poses a major challenge in the development and production of man-made materials with advanced functions. In contrast, for biologically formed hard tissues, this design feature and its formation principle are intrinsic. It began with the emergence of first skeletal hard parts in late Precambrian, with material construction principles being diversified since then by evolutionary adaptation for material functionality and survival in a wide range of environments and climates.
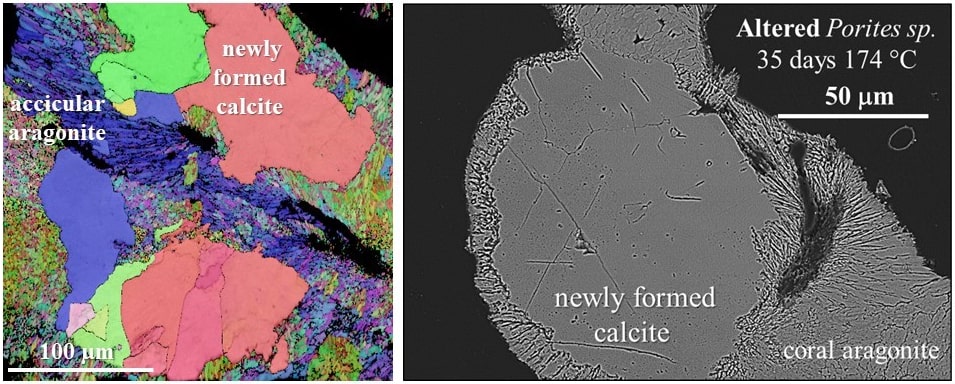
Response of carbonate micro- and nanostructures to diagenetic alteration
The assessment of diagenetic overprint on microstructural and geochemical data gained from fossil archives is of fundamental importance for understanding palaeoenvironmentsand paleoclimates. As fossilized structural materials are the archives of environment signals the correct reconstruction of past environmental dynamics is only possible when pristine skeletons are unequivocally distinguished from altered skeletal elements. Studies show that replacement of biogenic carbonate by inorganic calcite occurs via an interfacecoupled dissolution–reprecipitation mechanism. Thus a comprehensive understanding of alteration of the biogenic skeleton is only given when structural changes are assessed on both, the micrometre as well as on the nanometre scale.
Characterization of structural materials: biological and biomimetic carbonate composites
The main feature of the mineralization environment of biological carbonate hard tissues is an extracellular-matrix that consists of a three-dimensional assembly of proteins, polysaccharides, and glycoproteins. Hydrated networks of these biopolymers compartmentalize space and thus control morphology, functionalities, which are the template for carbonate crystal nucleation, and induce misorientation between nanoscale units which assemble to biocrystals such as nacre tablets or calcite fibers. Hydrogels mimic to some extent biological extracellular matrices, since the local crystallization microenvironment in the hydrogel is distinguished from that in solution by confinement of solutes in the pores of the hydrogel network. Hydrogels are regarded to model biogenic matrix environments due to their ability to confine space, to determine diffusion rates, and thus local concentrations or supersaturation of solutes. Hydrogels can be tailored to mimic characteristics of polymer matrices in biological hard tissues. However, hydrogels comprise synthetic compounds and lack attributes of native biopolymers, in contrast to extracellular-polymeric substance (EPS) actively secreted by microbes for protection and enhancement of physiological activities. The influence of bacterial EPS on mineralization and mineral organization is not at all as small as previously thought. In both, biologically controlled and biologically induced/influenced mineralization, the influence of the organism on the mineral formation process is substantial; the processes that lead to the biomineralized products differ significantly.